The poor tolerance and long-term adherence to CPAP, as well as the limitations of mechanical devices and surgery, make discovery of therapeutic alternatives clinically relevant and important. RespireRx’s translational research results demonstrate that dronabinol has the potential to become the first drug treatment for this large and underserved market.
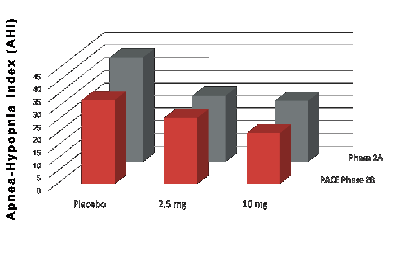
Based on the preclinical studies of Dr. David Carley of the University of Illinois Chicago (UIC), RespireRx conducted a 21-day, randomized, double-blind, placebo-controlled, dose escalation Phase 2A clinical study in 22 patients with OSA, in which FDA approved and commercially available dronabinol produced a statistically significant reduction in AHI, the primary therapeutic end-point. It was observed to be safe and well tolerated, with the frequency of side effects no different from placebo. This clinical trial provided data supporting the submission of patent applications claiming unique dosage strengths, blood levels and controlled release formulations optimized for use in the treatment of OSA. If approved, these pending patents would extend market exclusivity until January 2042.
Subsequently, Dr. Carley, along with his colleagues at UIC and Northwestern University, completed a Phase 2B multi-center, double-blind, placebo-controlled clinical trial of FDA approved and commercially available dronabinol in patients with OSA. This study, named “Pharmacotherapy of Apnea with Cannabimimetic Enhancement” (“PACE”) replicated our earlier Phase 2A study. The authors published in the January 2018issue of the journal SLEEP and reported that, in a dose-dependent fashion, treatment with 2.5 mg and 10 mg of dronabinol once per day at night significantly reduced, compared to placebo, AHI during sleep in 56 evaluable patients with moderate to severe OSA who completed the study. Additionally, treatment with 10 mg of dronabinol significantly improved daytime sleepiness as measured by the Epworth Sleepiness Scale and achieved the greatest overall patient satisfaction. As in our previous Phase 2A study, dronabinol was observed to be safe and well tolerated, with the frequency of side effects no different from placebo.
Based on the significance of these studies, we intend to continue development of dronabinol for the treatment of OSA, with the intention of submitting a 505(b)(2) New Drug Application (NDA) to the FDA.
